
Fiona Tolich checks the list in her exercise book for a second time, making sure there's a blue tick beside each child's name. Then she tallies the names out loud, her count barely audible as her eyes run down the page.
There are 21 names so far. She needs permission from each family if their child's photo is to be included in this story.
The first photograph arrives by email at 5.21pm on Sunday. "I give permission for Lincoln (age: 2.5) and Harlan (age: 10 months) to be featured."
The next one arrives four minutes later. Tama is smiling from his wheelchair, perched beside a water foundation. "I give permission for Tama (aged four) to be featured." Next comes permission for Liam, Skylah-Rose and Maia.
The emails continue over the weekend. Photos of Ivy and Olive and Heath and Logan. Toothy smiles, ice creams and days at the beach. Taylor and Charlotte and Ryan and Ava. Dress ups, a day at the zoo, a hospital bed.
One photograph cannot be used. "She is one of the children that tragically passed away," Tolich warns. "Please remove her photo from your files as her family will not want it included."
These kids are among about 35 New Zealand children with Spinal Muscular Atrophy (SMA), a severe, neurological condition which causes weakness and muscle wasting.
It's the most common cause of genetic death in infants. Without treatment those with the most serious form of SMA die before their second birthday.
There is a drug that vastly improves their condition - so much so that in trials nearly three-quarters of two year olds might look as healthy as the child who smiles at you from the screensaver on your cell phone. As healthy as the kids on that list of names you drew up for your daughter's birthday party.
Fiona Tolich has made it her life's mission to make the SMA drug available in New Zealand - as it is in Australia and 56 other countries.
She never expected her fight to end like this. Well, It's not over, but she is reeling - and just when she thought she had finally found the key.
Tolich had stumbled across a little known loophole in New Zealand's medicine rules, buried at the end of a highly technical Pharmac report - one of many the drug funding agency has produced over the three years it has been considering whether to fund the SMA drug.
The reference in the Pharmac report wasn't about kids with SMA but kids with cancer. It said this: "Hospitals may give, and will be eligible to receive a subsidy for, any pharmaceutical for use within a paediatric oncology/haematology service for the treatment of cancer."
So kids with cancer can get any drug their doctor thinks they need. Tolich had already taken a case under the Human Rights Act claiming discrimination against children with SMA and now it looked like she had an avenue to ensure her complaint was a slam dunk.
"Why would you pick kids with cancer to live and pick kids with SMA to die," she asks.
But in April, nearly a year after her complaint was lodged, the Office of Human Rights Proceedings warned her to be careful what she wished for.
The result may be that Pharmac would 'level the playing field' by decreasing access to child cancer drugs. "That would be a very disappointing outcome but it is one Pharmac has suggested it is willing to do."
Tolich felt her plea for SMA children to be granted access to life saving medicine was being met with a threat to take treatment away from children with cancer.
"I felt like I was being forced into a situation where there would be blood on my hands."

Fiona Tolich is so engrossed in her quest to get medicines for children with SMA - the application with Pharmac is for children 18 and under - that she sometimes forgets that she has SMA herself.
"I block it out a lot because I spend so much time fighting for the kids," she says. "I can't feel bad about what I've got knowing that, although I didn't win any genetic lottery, in a way I am so fortunate because it could have been a heck of a lot worse."
The impact of SMA varies wildly depending on when you get it. Prenatal onset leads to death within weeks. Without treatment an infant with a severe case of SMA is lucky to see their second birthday. If the condition comes on in adulthood you may have mild motor impairment - a loss of strength in the arms or legs - but a normal lifespan.
Tolich was diagnosed with SMA at 30 years old. She loves sport but running and softball had to fall by the wayside. "I'd get to first base and then I'd need a runner and I couldn't bat that well either!"
So she knew her limitations but mostly the rare genetic illness was a mystery to her.
"When you got diagnosed here, you got diagnosed and dumped basically: here's your diagnosis, and then nothing," she says. "I hadn't met anyone like me in New Zealand."
So in 2018 she travelled to a SMA conference in the United States. It was something of a pilgrimage, and in her eyes, what she saw was something approaching a miracle.
Kids with SMA who had lost the ability to crawl were walking. Here were children who had been facing a death sentence but were now living near normal lives.
"It was just amazing and so I came back thinking 'gosh, there's hope for us,' knowing that New Zealand was kind and caring. We were just waiting for the treatment to come here."
By the time Tolich travelled to America it had been two years since the FDA had approved Nusinersen, which has the brand name Spinraza, a treatment injected into the fluid around the spinal cord.
Dr Gina O'Grady, a paediatric neurologist at Starship Hospital in Auckland, describes Spinraza as "remarkable" and the results of clinical trials as "overwhelming".
Without treatment, children with Type 1 SMA, the most common and most serious form of the disease, die at a median age of 13 months. In New Zealand paediatric neurologists have cared for seven children with Type 1 SMA between 2014 and 2019. All have died.
"They would normally die because of weakness of their breathing. The muscles are not strong enough to sustain life," O'Grady explains.
At two years old 72 percent of treated infants did not show any clinical manifestations of SMA, according to the Nurture trial, involving 25 children.
"The majority of them are developing like healthy, typically developing infants, meeting their motor milestones at the age that you would expect them to," O'Grady says.
Those showing signs of weakness were only mildly affected.
"It's a dramatically effective drug. It's life changing for individuals who can get access to it."
And there's the catch. Spinraza, marketed by Biogen and developed by Ionis Pharmaceuticals, costs about $390,000 a year. In Australia parents pay $41 for it - or $6.60 a script - because it is publicly funded.
In New Zealand, Pharmac has been considering an application to fund Spinraza since August 2018 and while its own expert advisory committee ranked it as a "high priority" in February 2020 Pharmac still won't pay for it.
Time is vital with SMA. Every day lost is motor neurons lost that cannot be regained.
"The earlier you can start treatment the better your outcome. So the best case scenario is in countries where there is a newborn screening programme and so you pick up affected infants at birth and can start treatments in the first month," O'Grady says. "Those individuals can have a near normal life."
Spinraza does not cure SMA. "If you're later in the course of disease, and you've already lost a lot of your motor nerves, that treatment can't bring back the nerves that are lost. It's about slowing and preventing the loss of motor nerves, which is why we need to get in really early."
Biogen estimates the cost of adding SMA to newborn screening tests at $2.60 per live birth. New Zealand doesn't have newborn screening for SMA because without a publicly-funded medicine available it is seen as unethical to screen for it.
Professor Monique Ryan, Director of the Department of Neurology at the Royal Children's Hospital in Melbourne, says Spinraza has revolutionised the treatment of SMA in Australia.
"Our children with SMA type 1 can now confidently expect a long life with increasing evidence that very early treatment almost entirely eradicates clinical manifestations of this disorder," she wrote in a submission to New Zealand's health select committee in 2019.
"I can only guess how difficult it must be for parents of children with SMA in New Zealand knowing that their equivalents in Australia are able to access this medication for their affected children."
Fiona Tolich doesn't need to guess. She knows many of the families of children with SMA. Often they called her on the day their child was diagnosed. She's seen their photos and videos. She has been to funerals.
She'd written to the Prime Minister and the Health Minister. She'd made submissions to Pharmac and to select committees. Then a very different disease came along. It closed the schools, the workplaces and the borders. The government unleashed billions of dollars to save lives. The team of five million rowed in unison. But Tolich was watching from the sidelines.

In February 2020, just before New Zealand entered Covid-19 lockdown, Pharmac's advisory committee, PTAC, recommended Spinraza be funded as a high priority medicine.
Tolich knew enough about the system not to celebrate. There are currently 110 medicines recommended for funding by PTAC which Pharmac still doesn't fund and where they are ranked on the list is secret.
Her frustration was mounting. "The only option to preserve life right now is to go overseas and get access," she says. "Every day is potentially another neuron lost. And that might be the ability to sit, to stand, to walk, to swallow or even to breathe."
When Tolich saw the billions being shovelled out the door in response to Covid-19 - which she believes is warranted - the mild mannered former primary school teacher turned recruitment consultant went from frustrated to angry.
In May 2020, she lodged her complaint with the Human Rights Commission alleging discrimination against children with SMA. Nearly a year later, in April 2021, she had her reply.
Michael Timmins, the director of human rights proceedings, did not think her claim against Pharmac under the Human Rights Act was likely to be successful and so would not grant her legal representation for the case.
He stressed that wasn't because of the factual basis underpinning the claim but rather the weakness of the human rights framework in New Zealand.
"In paediatric cancer cases Pharmac funds all drugs requested by the patient's oncologist," Timmins wrote. "That creates a possible argument that Pharmac discriminates against SMA patients as compared to people with cancer".
But then came the warning.
"There is the risk that the answer to any discrimination claim would be to normalise the situation by decreasing paediatric cancer patients' access to treatment funding. That would be a very disappointing outcome but it is one Pharmac has suggested it is willing to do."
What Tolich didn't know was Pharmac had been considering for some time ending its blanket coverage for child cancer drugs - a situation that developed by historical accident.
Traditionally the budget for cancer drugs sat with district health boards (DHBs) but in 2005 the government began the process of transferring it over to Pharmac.
In 2010 cancer treatments were finally fully merged into the main Pharmac budget but complexities with child cancer drugs remained. Some of these drugs were provided in the context of clinical trials and other medicines were not registered for paediatric use.
The solution was to leave child cancer drugs with DHBs and create a funding exception for them under the Pharmac Schedule.
The upshot is hospitals are allowed to use any cancer drug to treat children and Pharmac will pay. The money comes from the main Pharmac budget and which drugs are provided is left to the clinician's judgement.
But now that is likely to change.

Pharmac Chief Executive Sarah Fitt says the situation is inconsistent and Pharmac will soon consult with its expert cancer committee and paediatric oncology doctors about changes.
The most likely outcome is paediatric cancer treatment decision making will be brought into Pharmac's usual processes.
She says there were about 50 children a year using medicines that were granted under the special exception rules and Pharmac spent only about $2 million of its $1 billion budget on those kids.
Fitt stresses Pharmac will not take any medicines away.
"We want to ensure that all the medicines currently being used will continue to be available and no medicines currently funded will be removed."
But she says new paediatric oncology drugs may be affected.
Asked if the new strategy could mean a decrease in access to paediatric oncology drugs, she says: "That may well be the outcome. But that is not our intent. We may leave it exactly as it is, but we need to actually do the work and talk to the people involved and not preempt any decision."
Fitt confirms it was Fiona Tolich's human rights complaint that finally jolted Pharmac into taking action.
"This complaint has highlighted that inconsistency and we committed to Fiona and to the Human Rights Commission that we would look at it because it wasn't consistent. It's something we've wanted to do but we haven't managed to do it in the last few years."
While Pharmac sees it as an inconsistency it is finally getting around to ironing out, Tolich sees it as a punishment or at least a threat.
"Everything I've done so far has been about helping and fighting for fairness, and fighting for access and fighting for life," she says. "I felt like I was put in a position where I might be doing the opposite of that for one group of patients, and a very young group of patients. And that made me feel sick."
At the moment there are eight children with Type One SMA in New Zealand but we effectively outsource their treatment to a drug company.
Biogen runs a compassion scheme offering Spinraza to Type One SMA kids for free. But there are only 10 places and three or four children with Type One SMA are born in New Zealand each year.
"It would be absolutely devastating to have to go back to telling families that their child will die, that we've got no treatment available in New Zealand," Starship's Gina O'Grady says.
Biogen's Managing Director for Australia and New Zealand Kylie Bromley told RNZ the company had gone back to Pharmac seven times in the last two years - each time offering a lower price.
"We understand that this delay has put approximately 35 children at risk of significant functional decline and even possibly death during this delay period."
Bromley said the compassion scheme was designed as a life saving measure for SMA babies while public funding was being sought and never as a replacement for the role of the health service.
A letter the company sent to Tolich in July 2020 was even more blunt.
"Due to our experience with Pharmac we were unsurprised to recently learn that New Zealand remains in last place out of 20 comparable OECD countries for access to modern medicines which is at odds with the picture the country paints of itself in terms of being an equitable society that cares for its vulnerable."
That lecture may ring hollow with some given the company charges about $390,000 a year for this medicine. How can it justify that? Bromely says Biogen sets the price based on the innovation and research costs it spent developing the drug and the value to the patient and to wider society.
The cost of Spinraza is similar to that of Kalydeco, a treatment for Cystic Fibrosis, which Pharmac agreed to fund in January 2020 - six years after the application was made.
While Spinraza has a 'list price' of about $390,000 a year, drug industry experts estimate Pharmac often secures a 50 percent discount through its confidential negotiating process. On that basis it would cost about $7 million a year to provide 35 children with Spinraza.
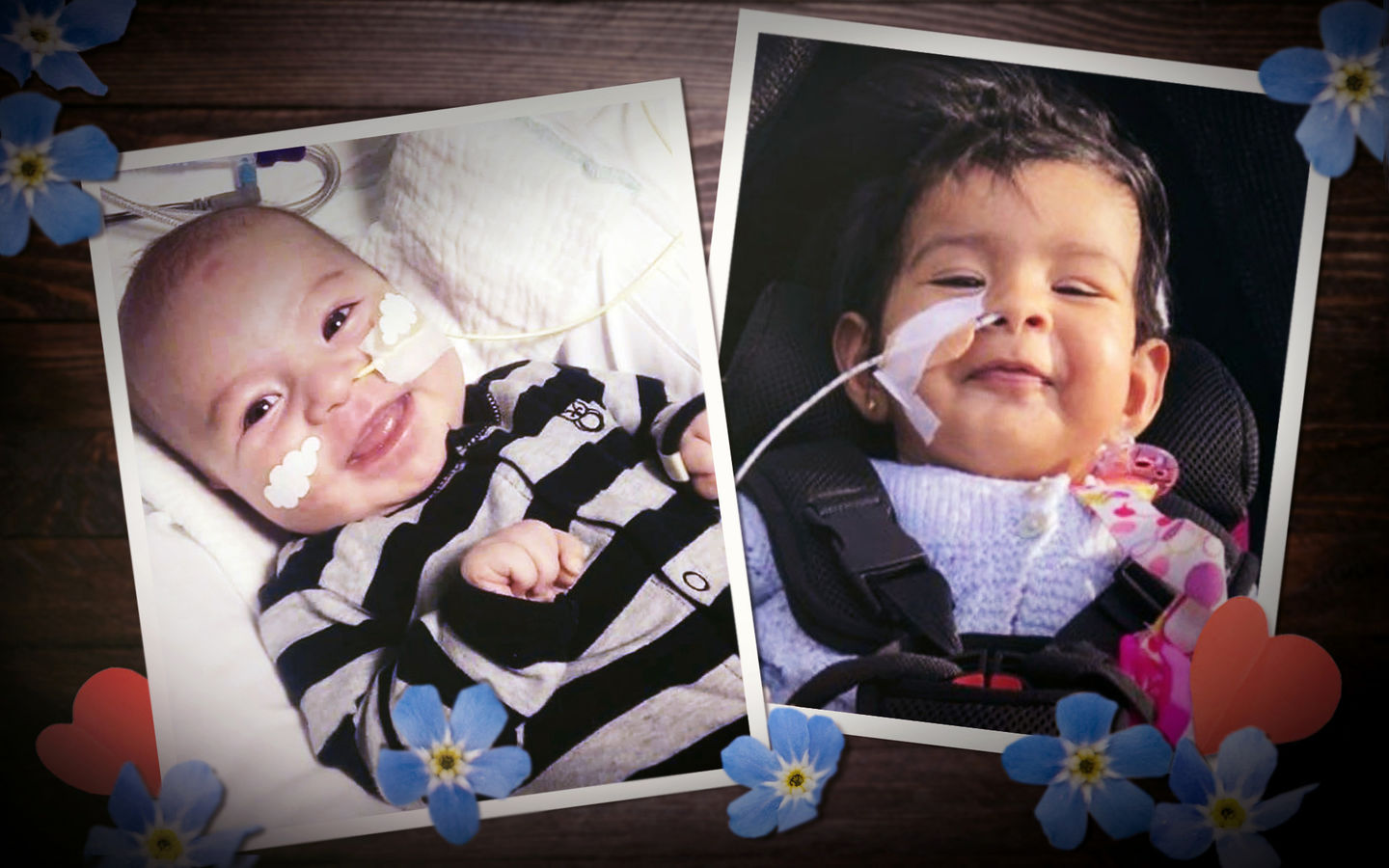
The photographs of the children keep coming in the week after I visit Tolich at her home.
I have created my own list now, my own blue ticks against the name of each child. There are two columns in my notebook. In the left column are the names of 22 children who are still alive. In the right column are the names of two children who have died. The families want those two photos used too.
Tolich doesn't want any more children to move from the left column to the right.
"I'm looking at photos that parents send me after the kids have passed away," she says. "I've attended funerals of kids who have passed away and these are kids that could be saved with a treatment that does what it says it does. The rest of the world is seeing that."
Tolich feels she has exhausted most of her options in New Zealand now so next she will take the fight abroad, perhaps to the United Nations.
"We've long portrayed ourselves as being the best place in the world to raise a child. I can't see how we can stand by that when we're allowing our country to pick and choose which ones live in which ones die when there's treatment available for both."












